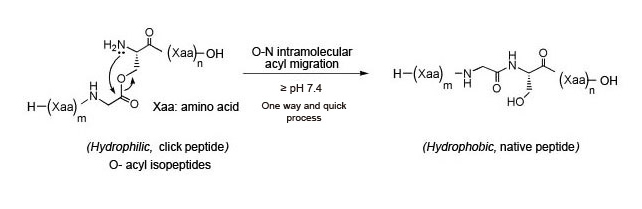
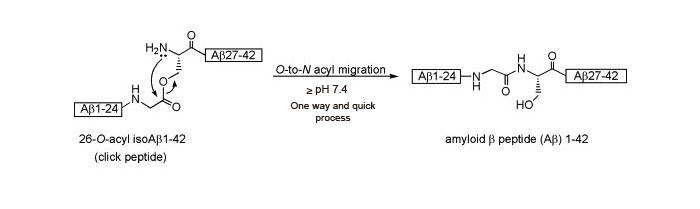
Atsuhiko Taniguchi, Youhei Sohma, et al. Click peptide: Chemical Biology-oriented analogues of Alzheimer's amyloid β peptide 1–42. J. Peptide Science. Nov 2006; 12(12): 823-828
Youhei Sohma, Atsuhiko Taniguchi, et al. Controlled Production of Amyloid β Peptide from a Photo-Triggered, Water-Soluble Precursor "Click Peptide". ChemBioChem. Nov 2008; 9(18): 3055-3065
Hui Wang, Taeko Kakizawa, et al. Synthesis of amyloid β peptide 1–42 (E22Δ) click peptide: pH-triggered in situ production of its native form. Bioorganic & Medicinal Chemistry. July 2009; 17(14): 4881-4887
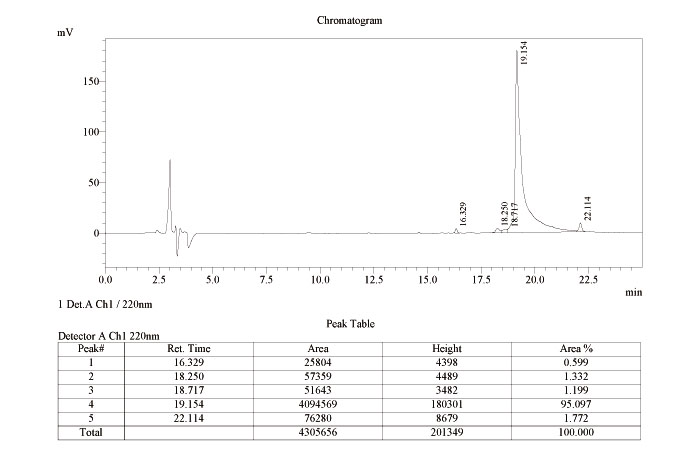
Taniguchi A, Kiso Y, et al. "Click peptide": pH-triggered in situ production and aggregation of monomer Abeta1-42. ChemBioChem. Mar 2009; 10(4): 710-715
Claudia Balducci, Marten Beeg, et al. Synthetic amyloid-β oligomers impair long-term memory independently of cellular prion protein. PNAS. Jan 2010; doi:10.1073